Leeds mum's joy after anxious wait over drugs trial for four-year-old daughter with potentially fatal condition
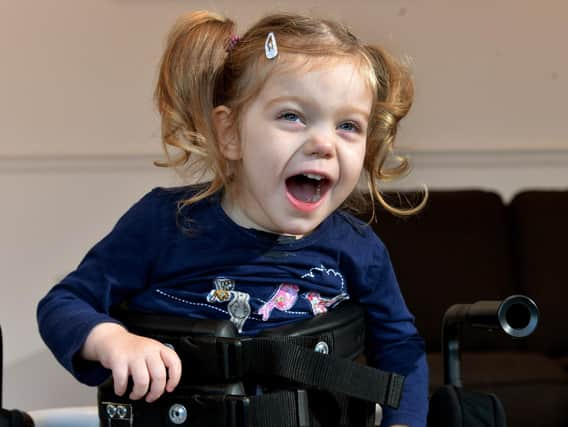

Coronavirus travel restrictions meant Zoe Lightfoot of Bramley was unable to travel to America in June for tests during the drug trial, which she has been on for 18-months.
Zoe has infantile neuroaxonal dystrophy (INAD), which usually means an affected child dies between the ages of five and 10.
Advertisement
Hide AdAdvertisement
Hide AdZoe's mum Christine Hamshere said she was down to the last few tablets of the drug when a fresh three-month supply arrived from the USA on Wednesday (August 5).


And the drug company is arranging with Zoe's neurologist at Leeds General Infirmary for tests and assessments for the trial - which are usually done in America - to take place at Leeds General Infirmary.
Miss Hamshere said: "It is such a relief. We are really happy that she can continue with the medication because we do feel it is helping her.
"We have been given hope. We are delighted that she can continue with the trial as there is nothing else out there at the moment - without this we would lose hope."
Advertisement
Hide AdAdvertisement
Hide AdSince the diagnosis two years ago, Zoe has lost mobility and her speech.
Zoe and her family have been to the Goryeb Children’s Hospital in Morristown, New Jersey in America four times since November 2018 as part of a clinical drugs trial it is hoped will curb her decline.
They were due to return to the hospital for a six-day stay at the end of June and start of July to continue with the Retrotope drug company trial.
But the trip was cancelled and the family was anxiously waiting to hear if the drug would be sent to the UK so the trial can continue.
Advertisement
Hide AdAdvertisement
Hide AdZoe is one of only 19 children worldwide picked for the trial and since launching a fundraising effort, parents Christine Hamshere and Steven Lightfoot have seen people donate more than £28,000.
The money is paying for Zoe's treatment and is helping charity the INADcure Foundation, which is trying to find a cure for INAD.
Miss Hamshere, who also has a 16-month-old baby son called Alex, said she believes the trial drug is increasing Zoe's life expectancy and is giving her a better quality of life.
According to the Great Ormond Street Hospital, which specialises in care for children, INAD is a rare inherited disorder affecting the nerve axons which are responsible for conducting messages in the brain and other parts of the body.
Advertisement
Hide AdAdvertisement
Hide AdZoe was diagnosed in April 2018 after an MRI brain scan in January that year revealed significant brain damage and cerebellar atrophy.
The part of the brain that affects balance and coordination, speech and swallowing had formed normally as a baby, but then shrivelled.
It causes a progressive loss of vision and of physical and mental skills.
Visit the fundraising page at https://www.gofundme.com/zoes-inad-battle-fund
Advertisement
Hide AdAdvertisement
Hide AdA message from the Editor: Leeds has a fantastic story to tell - and the Yorkshire Evening Post has been rooted firmly at the heart of telling the stories of our city since 1890. We believe in ourselves and hope you believe in us too. We need your support to help ensure we can continue to be at the heart of life in Leeds. Subscribe to our website and enjoy unlimited access to local news and information online and on our app. With a digital subscription, you can read more than 5 articles, see fewer ads, enjoy faster load times, and get access to exclusive newsletters and content. Click here to subscribe. For more details on our newspaper subscription offers click here.
Thank you
Laura Collins
Comment Guidelines
National World encourages reader discussion on our stories. User feedback, insights and back-and-forth exchanges add a rich layer of context to reporting. Please review our Community Guidelines before commenting.